Fuels produced in harmony with nature.
Technology
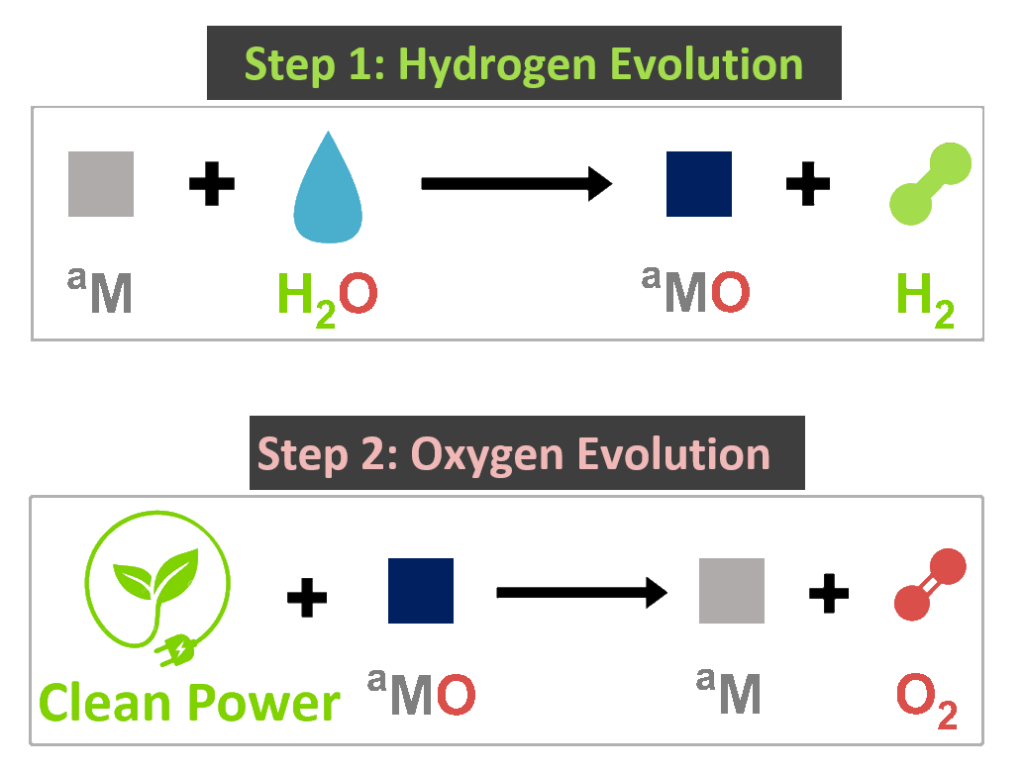
We have developed an innovative two-step water electrolysis method that uses Hydropore’s activated Earth-abundant metals (aM) to split water molecules and release the hydrogen and oxygen contained within them.
During the first step of our process (the hydrogen evolution step), our activated metals naturally react with water to produce hydrogen gas and the corresponding metal oxide as the solid by-product.
In the second step (the oxygen evolution step and renewable energy storage step), clean electricity is used to electrochemically release oxygen from the metal oxide, thereby converting the metal oxide back to activated metal for reuse. This step stores renewable energy in metals for future use.
Advantages of Hydropore’s solution:
- 24/7 Clean Hydrogen: Our water-splitting technology decouples hydrogen production and electricity consumption. Electricity can be consumed and stored during hours when renewable energy is produced and used to produce clean hydrogen at any time thereafter. This allows for high electrolyzer utilization, even when paired with intermittent renewable energy production.
- Energy Cost Savings: Two-step electrolysis allows for more effective energy cost arbitrage, as electricity can be consumed and stored when wholesale prices or peak demand charges are lowest.
- No Membranes or Noble Metals: No need for proton and anion exchange membranes to separate hydrogen and oxygen, since these two gases are not produced at the same time like with conventional electrolyzers.
- Water Requirement: No need for high-purity water to produce hydrogen. Since our membrane-free water-splitting technology is simply based on the oxidation and reduction of Earth-abundant metals, any water source with a neutral or moderate pH can be used to produce hydrogen.
- Efficient Reaction: No need to capture the heat generated from metal-water reactions to maximize the process efficiency. Our selected metals are mildly exothermic upon reaction with water, with over 85% of the reaction energy converted to hydrogen.